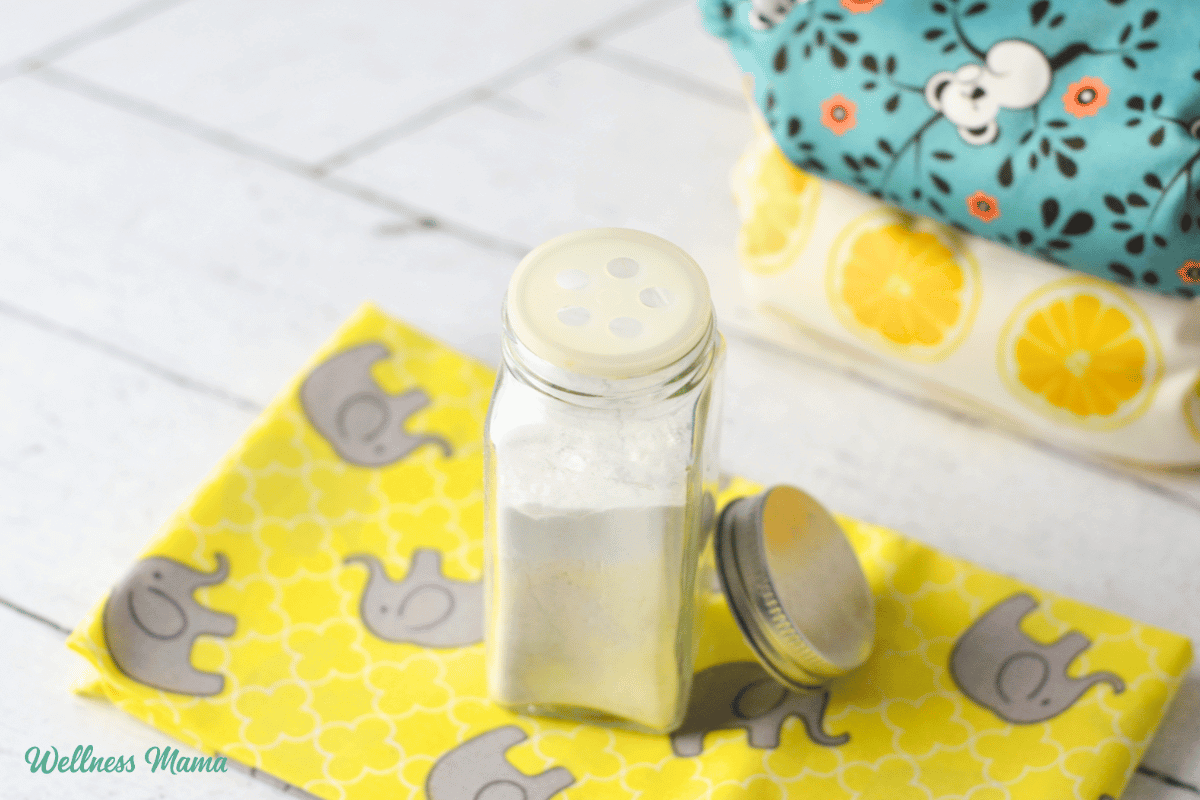New committee members Dr. Robert Malone, left, and Dr. Joseph Hibbeln through the first assembly of the CDC’s Advisory Committee On Immunization Practices on the Facilities for Illness Management and Prevention in Atlanta, Ga.
Ben Hendren/Bloomberg/Getty Pictures
disguise caption
toggle caption
Ben Hendren/Bloomberg/Getty Pictures
An influential committee that shapes U.S. vaccine coverage – a flashpoint beneath the management of Secretary of Well being Robert F. Kennedy Jr. – has really useful that adults and kids now not obtain flu vaccines containing hint quantities of a preservative that is not often used anymore.
The dialogue of thimerosal, a type of mercury that is generally added to vaccines for sterilization, dominated a lot of Thursday’s public assembly of the Advisory Committee on Immunization Practices, or ACIP. The committee guides the Facilities for Illness Management and Prevention on the vaccine schedule for youngsters and adults.
The 2-day assembly at CDC headquarters in Atlanta was unusually high-profile, given Kennedy’s current determination besides the complete committee of specialists just a few weeks in the past and substitute them with his personal hand-picked roster, which included some members with a historical past of constructing inaccurate claims in regards to the security of vaccines.
Whereas ACIP usually contains 17 voting members, Kennedy’s overhauled panel solely included seven of them, following a last-minute determination by one in all them to step down.
On Thursday, a majority of the panel voted to reaffirm the prevailing CDC suggestions that anybody over six months obtain the annual flu shot. In addition they voted 5-2 in favor of a monoclonal antibody shot made by Merck that gives safety towards respiratory syncytial virus, or RSV, for infants youthful than 8 months.
However in three separate votes the committee voted to advocate youngsters, pregnant ladies and all adults obtain single-dose flu immunizations with vaccines that do not have thimerosal.
Theories that the chemical may trigger autism in youngsters have lengthy been disproven. Even so, producers voluntarily eliminated it from childhood vaccines. Whereas it is used in some multi-dose vials in a number of merchandise, there are not any vaccines on the pediatric vaccine schedule that include thimerosal.
The ACIP votes may successfully ban use of the preservative, regardless of a preponderance of proof that it’s protected.
Dr. Cody Meissner, a professor of pediatrics at Dartmouth School, was the one ACIP member who voted towards these suggestions.
“Of all the problems that ACIP must deal with, this isn’t an enormous situation,” he mentioned. “The danger from influenza is a lot larger than the non-existent, so far as we all know, threat from thimerosal.”
He added: “There isn’t any scientific proof that thimerosal has brought about an issue.”
Meissner’s feedback got here in response to a prolonged presentation on the preservative from Lyn Redwood, a nurse and former president of Kids’s Well being Protection, the anti-vaccine advocacy group that Kennedy based and led for a few years.
“Eradicating a recognized neurotoxin from being injected into our most weak inhabitants is an efficient place to begin with making America wholesome once more,” she advised the committee.
A lot of what Redwood mentioned about thimerosal was undercut by a CDC doc that had been initially posted with the assembly supplies — after which with out rationalization eliminated forward of the assembly. It detailed peer-reviewed literature displaying “no affiliation between prenatal publicity to thimerosal-containing vaccinations and autism spectrum dysfunction in youngsters.”
In response to a query about why the doc had been taken down, Dr. Robert Malone, an ACIP member, mentioned his understanding was that it had not been “licensed by the Workplace of the Secretary.”
Members of the committee questioned the analyses offered by CDC, and puzzled whether or not they had been ignoring information on hostile occasions that confirmed up in relation to the research they offered, even after the employees defined they’d parsed the information completely.
Outstanding medical teams welcomed the suggestions on flu and RSV, however expressed concern in regards to the general tone of the assembly.
Dr. Sean O’Leary, who chairs the Committee on Infectious Ailments for the American Academy of Pediatrics, mentioned the ACIP dialogue on influenza and RSV “confirmed that that is an orchestrated effort to sow mistrust in immunizations and the vaccine approval course of.”
Whereas the particular person serving as CDC director would usually log off on ACIP suggestions, there is no such thing as a one within the function at the moment, so the duty for signing off on these suggestions go to Well being Secretary Kennedy.